7.1 Chemical Equations
Word Equation: Everything in full English.
Chemical Equations: Symbols of elements.
7.2 Ionic Equations
Ionic Equations: Symbols of elements, charge of element and state of elements.
Spectator ions are not to be included in the ionic equations.
Spectator ions are ions still in solution at the end of the reaction.
Friday, October 30, 2009
Chemistry: Chapter 9 - Chemical Calculations
9.1 Calculations from Chemical Reactions
A balanced chemical equation shows the important facts about a reaction:
9.2 The Volumes of Reacting Gases
Volume of a gas is proportional to the number of moles of the gas, and vice versa.
9.3 Limiting Reactants
The reactant that is completely used up in a reaction is known as the limiting reactant.
The reactants that are not used up are called the excess reactants.
9.4 The Concentration of a Solution
The concentration of a solution is given by the amount of a solute dissolved in a unit volume of a solution.
Concentration (g/dm3) = Mass of Solute in Grams / Volume of Solution in dm3
Molar Concentration
Concentration (mol/dm3) = number of moles of solute / volume of solution in dm3
Concentration (mol/dm3) = concentration (g/dm3) / Molecular mass of solute
9.5 Volumetric Analysis
Volumetric analysis is a technique used to determine the volumes of solutions that react together. In volumetric analysis, titration is performed to get the results.
A balanced chemical equation shows the important facts about a reaction:
- The reactants
- The products
- The ration of the amounts (in moles) of the reactants and the products
- The state of the reactants and products indicated.
9.2 The Volumes of Reacting Gases
Volume of a gas is proportional to the number of moles of the gas, and vice versa.
9.3 Limiting Reactants
The reactant that is completely used up in a reaction is known as the limiting reactant.
The reactants that are not used up are called the excess reactants.
9.4 The Concentration of a Solution
The concentration of a solution is given by the amount of a solute dissolved in a unit volume of a solution.
Concentration (g/dm3) = Mass of Solute in Grams / Volume of Solution in dm3
Molar Concentration
Concentration (mol/dm3) = number of moles of solute / volume of solution in dm3
Concentration (mol/dm3) = concentration (g/dm3) / Molecular mass of solute
9.5 Volumetric Analysis
Volumetric analysis is a technique used to determine the volumes of solutions that react together. In volumetric analysis, titration is performed to get the results.
Thursday, October 29, 2009
Chemistry: Chapter 6 - Chemical Bonding
6.1 The Stable Noble Gas Structure
Atoms of noble gases are usually unreactive or stable.
An atom is stable if it has a duplet or octet configuration.
6.2 Forming Ions
An ion is a charged particle formed from an atom or a group of atoms by the loss or gain of electrons.
Metals form positively charged ions (cations) whereas non-metals form negatively charged ions (anions).
Metals give electrons and non-metals take electrons.
6.3 Ionic Bond : Transferring Electrons
When metals react with non-metals, an ionic compound is formed.
Positive ions and negative ions are attracted to one another by electrostatic attraction.
An ionic bond may also be known as an electrovalent bond.
Compounds that has ionic bonds are called ionic compounds.
Structure of Ionic Compounds
Ionic compounds are arranged in a giant lattice structure or crystal lattice.

Physical Properties of Ionic Compounds
When non-metals react with non-metals, an covalent bond is formed.
Structure of Covalent Bond Compounds


Physical Properties of Covalent Substances
*Ionic compounds have electrostatic attraction. Covalent compounds have intermolecular forces.
Atoms of noble gases are usually unreactive or stable.
An atom is stable if it has a duplet or octet configuration.
6.2 Forming Ions
An ion is a charged particle formed from an atom or a group of atoms by the loss or gain of electrons.
Metals form positively charged ions (cations) whereas non-metals form negatively charged ions (anions).
Metals give electrons and non-metals take electrons.
6.3 Ionic Bond : Transferring Electrons
When metals react with non-metals, an ionic compound is formed.
Positive ions and negative ions are attracted to one another by electrostatic attraction.
An ionic bond may also be known as an electrovalent bond.
Compounds that has ionic bonds are called ionic compounds.
Structure of Ionic Compounds
Ionic compounds are arranged in a giant lattice structure or crystal lattice.

Physical Properties of Ionic Compounds
- Ionic compounds have high melting and high boiling points. (Non-volatile substances)
- Ionic compounds are soluble in water but not in oil.
- Ionic compounds do not conduct electricity in the solid state but in the molten state as there are free-moving ions that conduct electricity.
When non-metals react with non-metals, an covalent bond is formed.
Structure of Covalent Bond Compounds


Physical Properties of Covalent Substances
- Covalent compounds have low melting and low boiling points.
- Covalent compounds are soluble in oil but now in water.
- Covalent compounds do not conduct electricity in any state.
*Ionic compounds have electrostatic attraction. Covalent compounds have intermolecular forces.
Saturday, October 24, 2009
Physics: Chapter 13 - Sound
13.2 Transmission of Sound
Sound waves need a medium in order to travel from one point to another.
13.3 Reflection of Sound
An echo is formed when a sound is reflected off hard, flat surfaces such as a large wall or a distant cliff.
Uses of echos
Use to detect the position of mines and submarines.
13.4 Pitch and Loudness
Pitch
Pitch is related to the frequency of a sound wave.
Sound with lower frequency has a lower pitch.
Sound with higher frequency has a higher pitch.
Loudness
Loudness is related to the amplitude of a sound.
The larger the amplitude, the louder the sound.
The shorter the amplitude, the softer the sound.
Physics: Chapter 12 - Electromagnetic Waves
12.1 Electromagnetic Waves
Ronald Mcdonald is very ugly X-cept Gary
Radio Waves - Longer wavelength Lower Frequency
Microwaves
Infrared
Visible light
Ultraviolet
X-rays
Ronald Mcdonald is very ugly X-cept Gary
Radio Waves - Longer wavelength Lower Frequency
Microwaves
Infrared
Visible light
Ultraviolet
X-rays
Gamma rays - Shorter wavelength High Frequency
Properties of electromagnetic waves
Application of elctromagnetic waves
Gamma Rays: Radiation therapy (Cancer treatment)
X-Rays: Medical and everyday application
Ultraviolet: Sunbeds and sterilisation of medical equipment
Visible light: Optical fibres
Infrared: Remote controllers and ear thermometers
Microwaves: Ovens and satellites
Radio waves: Radio and telecommunications
Effects of Electromagnetic Waves
Infrared heating - Skin absorbs infrared waves, making us feel warm.
Properties of electromagnetic waves
- Electromagnetic waves are transverse waves.
- They transfer energy from one place to another.
- They can travel through vacuum. Do not require any medium to travel around.
- They travel at a speed of 3.0 X 108 ms-1 in vacuum.
- They obey the laws of reflection and refraction.
- They carry no electric charge.
- Their frequencies do not change when they travel from one medium to another. Only their speeds and wavelengths change from one medium to another.
Application of elctromagnetic waves
Gamma Rays: Radiation therapy (Cancer treatment)
X-Rays: Medical and everyday application
Ultraviolet: Sunbeds and sterilisation of medical equipment
Visible light: Optical fibres
Infrared: Remote controllers and ear thermometers
Microwaves: Ovens and satellites
Radio waves: Radio and telecommunications
Effects of Electromagnetic Waves
Infrared heating - Skin absorbs infrared waves, making us feel warm.
Friday, October 23, 2009
Physics: Chapter 11 - Waves
11.1 Describing Waves
The source of a wave is vibration or oscillation.
Waves transfer energy from one point to another.
In waves, energy is transferred without the medium moving/ being transferred.
Transverse waves are waves that travel in a direction perpendicular to the direction of vibration of the particles.
 Examples of transverse waves: Light waves
Examples of transverse waves: Light waves
Longitudinal waves are waves that travel in a direction parallel to the direction of vibration of particles.

Examples of longitudinal waves: Sound waves.
11.2 Properties of Wave Motion

Crest and troughs: Highest and lowest points of a transverse waves.
Compression and rarefaction : Highest and lowest points of a longitudinal waves.
Phase: Points of a wave which move in the same direction, have the same speed and the same displacement from the original position.
Wavelength λ: Shortest distance between any two points in a wave that are in phase.
SI Unit: metre (m)
Amplitude A: Maximum distance from the rest position. It is the height of a crest or depth of a trough.
SI Unit: metre (m)

Period (T): Time taken for one point on the wave to complete one oscillation.
SI Unit: second (s)
Frequency (f): Number of complete waves produced per second.
SI Unit: Hertz (Hz)
f = 1/T
Wave speed (v): v = fλ
Wave speed = wavelength(period)
SI Unit: metre per second (m s-1)
Wavefront: Imaginary line on a wave that joins all points that are in the same phase.
The source of a wave is vibration or oscillation.
Waves transfer energy from one point to another.
In waves, energy is transferred without the medium moving/ being transferred.
Transverse waves are waves that travel in a direction perpendicular to the direction of vibration of the particles.
 Examples of transverse waves: Light waves
Examples of transverse waves: Light wavesLongitudinal waves are waves that travel in a direction parallel to the direction of vibration of particles.

Examples of longitudinal waves: Sound waves.
11.2 Properties of Wave Motion

Crest and troughs: Highest and lowest points of a transverse waves.
Compression and rarefaction : Highest and lowest points of a longitudinal waves.
Phase: Points of a wave which move in the same direction, have the same speed and the same displacement from the original position.
Wavelength λ: Shortest distance between any two points in a wave that are in phase.
SI Unit: metre (m)
Amplitude A: Maximum distance from the rest position. It is the height of a crest or depth of a trough.
SI Unit: metre (m)

Period (T): Time taken for one point on the wave to complete one oscillation.
SI Unit: second (s)
Frequency (f): Number of complete waves produced per second.
SI Unit: Hertz (Hz)
f = 1/T
Wave speed (v): v = fλ
Wave speed = wavelength(period)
SI Unit: metre per second (m s-1)
Wavefront: Imaginary line on a wave that joins all points that are in the same phase.
Physics: Chapter 9 - Thermal Properties of Matter
9.1 Temperature and Internal Energy
Internal energy is made up of kinetic energy and potential energy.
9.2 Melting and Solidification
Melting is the change of state from solid to liquid, without a change in temperature
During melting, the temperature remains constant at the melting point.
Thermal energy is absorbed by the substance.
Solidification is the change of state from liquid to solid, without a change in temperature.
During solidification, the temperature remains constant at the freezing point.
Thermal energy is released by the substance.
9.3 Boiling and Condensation
Boiling is the change of state from a liquid into vapour, occurring at a constant temperature called the boiling point.
During boiling, the temperature remains constant at its boiling point.
Thermal energy is being absorbed by the substance.
Condensation is the process whereby vapour changes into liquid at the same constant temperature. Heat is given out during condensation.
During condensation, the temperature remains constant at the condensation point. Thermal energy is released by the substance.
9.4 Evaporation
Factors affecting the rate of evaporation
Heating a liquid will increase the rate of evaporation because it means a greater number of molecules at the surface layer are energetic enough to escape.
2. Humidity of the surrounding air
Rate of evaporation decreases with increasing humidity (Water vapour present in the air).
3. Surface area of the liquid
Larger surface area = Increase in evaporation
4. Movement of air
Rate of evaporation increases when the surrounding air is moving.
Moving air removes the molecules of the liquid as soon as they escape from the surface of something.
5. Pressure
Reducing the atmospheric pressure increases the rate of evaporation.
Example: Things can dry faster on mountaintops than at sea level.
6. Boiling point of the liquid
Liquids with lower boiling point evaporates faster.
Internal energy is made up of kinetic energy and potential energy.
9.2 Melting and Solidification
Melting is the change of state from solid to liquid, without a change in temperature
During melting, the temperature remains constant at the melting point.
Thermal energy is absorbed by the substance.
Solidification is the change of state from liquid to solid, without a change in temperature.
During solidification, the temperature remains constant at the freezing point.
Thermal energy is released by the substance.
9.3 Boiling and Condensation
Boiling is the change of state from a liquid into vapour, occurring at a constant temperature called the boiling point.
During boiling, the temperature remains constant at its boiling point.
Thermal energy is being absorbed by the substance.
Condensation is the process whereby vapour changes into liquid at the same constant temperature. Heat is given out during condensation.
During condensation, the temperature remains constant at the condensation point. Thermal energy is released by the substance.
9.4 Evaporation
Factors affecting the rate of evaporation
- Temperature
- Humidity of the surrounding air
- Surface area of the liquid
- Movement of air
- Pressure
- Boiling point of the liquid
Heating a liquid will increase the rate of evaporation because it means a greater number of molecules at the surface layer are energetic enough to escape.
2. Humidity of the surrounding air
Rate of evaporation decreases with increasing humidity (Water vapour present in the air).
3. Surface area of the liquid
Larger surface area = Increase in evaporation
4. Movement of air
Rate of evaporation increases when the surrounding air is moving.
Moving air removes the molecules of the liquid as soon as they escape from the surface of something.
5. Pressure
Reducing the atmospheric pressure increases the rate of evaporation.
Example: Things can dry faster on mountaintops than at sea level.
6. Boiling point of the liquid
Liquids with lower boiling point evaporates faster.
Physics: Chapter 8 - Transfer of Thermal Energy
8.1 Transfer of Thermal Energy
Thermal energy always flows from a region of higher temperature to a region of lower temperature.
8.2 Conduction
Conduction is the process of thermal energy transfer without the medium flowing.
Occurs mostly in solids, good conductor of heat.
Thermal energy is supplied to a material -> Particles at hot end vibrate vigorously -> Particles collide with neighbouring particles -> Kinetic energy is transferred to the neighbouring particles.
This happens because metals contain many free electrons which move randomly between the atoms or molecules.
8.3 Convection
Convection is the transfer of thermal energy by means of currents in a fluid or gases.
When water at the bottom of a container is heated, it expands -> Expanded water is less dense and start to ride -> Cooler regions of the water in the upper part on container became denser and sinks.
Convection currents occur only in fluids such as liquids and gas but not in solids.
Because convection involves the bulk movement of the fluids which carry thermal energy with them.
8.4 Radiation
Radiation is the continual emission of infrared waves from the surface of all bodies, transmitted without the aid of a medium.
The process in which energy is emitted as particles or waves.
Radiation can take place in a vacuum.
Factors affecting rate if infrared radiation
Emission = give out
Best absorption and emission: dull black surface
Thermal energy always flows from a region of higher temperature to a region of lower temperature.
8.2 Conduction
Conduction is the process of thermal energy transfer without the medium flowing.
Occurs mostly in solids, good conductor of heat.
Thermal energy is supplied to a material -> Particles at hot end vibrate vigorously -> Particles collide with neighbouring particles -> Kinetic energy is transferred to the neighbouring particles.
This happens because metals contain many free electrons which move randomly between the atoms or molecules.
8.3 Convection
Convection is the transfer of thermal energy by means of currents in a fluid or gases.
When water at the bottom of a container is heated, it expands -> Expanded water is less dense and start to ride -> Cooler regions of the water in the upper part on container became denser and sinks.
Convection currents occur only in fluids such as liquids and gas but not in solids.
Because convection involves the bulk movement of the fluids which carry thermal energy with them.
8.4 Radiation
Radiation is the continual emission of infrared waves from the surface of all bodies, transmitted without the aid of a medium.
The process in which energy is emitted as particles or waves.
Radiation can take place in a vacuum.
Factors affecting rate if infrared radiation
- Colour and texture of the surface
- Surface temperature
- Surface area
Emission = give out
Best absorption and emission: dull black surface
Physics: Chapter 7 - Kinetic Model of Matter
Matter are tiny particles which are always in continuous, random motion.
Solid
Gas particles move faster at higher temperature due to a increase of thermal energy which is converted to kinetic energy of the air molecules.
Solid
- Closely packed atoms or molecules
- Strong intermolecular bonds
- Atoms or molecules vibrate about fixed positions.
- Atoms or molecules occur in clusters
- Slightly further apart compared to solids
- Free to move about between clusters
- Atoms or molecules are very far apart
- Negligible attractive forces between atoms or molecules
- High speed, independent motion in random manner
Gas particles move faster at higher temperature due to a increase of thermal energy which is converted to kinetic energy of the air molecules.
Physics: Chapter 6 - Energy, Work and Power
6.1 Energy
Energy is the capacity to do work.
SI Unit: joule (J)
Types of energy:
1. Kinetic Energy
Moving objects have kinetic energy.
2a. Potential Energy - Chemical Potential Energy
Can be found in food, fossil fuels.
2b. Potential Energy - Elastic Potential Energy
A spring or rubber band possesses elastic potential energy when compressed or stretched.
When released, it is converted into kinetic energy.
2c. Potential Energy - Gravitational Potential Energy
The higher the object that leaves the ground, the greater the gravitational potential energy.
Principle of Conservation of Energy
Efficiency = useful energy output divide energy input times 100%
6.2 Work
W=F(s)
Work done by a constant force = constant force(distance moved by the object in the direction of the force)
No work is done when:
Mechanical Energy
1. Kinetic energy and work done
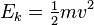 Kinetic energy = 1/2(mass of body)(speed of body)²
Kinetic energy = 1/2(mass of body)(speed of body)²
2. Gravitational potential energy and work done
Ep = mgh
Potential energy = mass(gravitational field strength)(height)
6.3 Power
P= W/t = E/t
Power = work done over time = energy converted over time
SI Unit: watt (W)
Energy is the capacity to do work.
SI Unit: joule (J)
Types of energy:
- Kinetic Energy
- Potential Energy (Chemical potential, elastic potential and gravitational potential)
1. Kinetic Energy
Moving objects have kinetic energy.
2a. Potential Energy - Chemical Potential Energy
Can be found in food, fossil fuels.
2b. Potential Energy - Elastic Potential Energy
A spring or rubber band possesses elastic potential energy when compressed or stretched.
When released, it is converted into kinetic energy.
2c. Potential Energy - Gravitational Potential Energy
The higher the object that leaves the ground, the greater the gravitational potential energy.
Principle of Conservation of Energy
Energy can neither be created nor destroyed in any process. It can be converted from one form to another or transferred from one body to another, but the total amount remains constant.Conversion of energy
Efficiency = useful energy output divide energy input times 100%
6.2 Work
Work done by a constant force on an object is given by the product of the force and the distance moved by the object in the direction of the force.
W=F(s)
Work done by a constant force = constant force(distance moved by the object in the direction of the force)
One joule is defined as the work done by a force of one newton which moves an object through a distance of one metre in the direction of the force.
No work is done when:
- The direction of the applied force and the direction in which the object moves are perpendicular to each other, and
- The force is applied on the object but the object does not move.
Mechanical Energy
- Kinetic energy and work done
- Gravitational potential energy and work done
1. Kinetic energy and work done
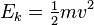 Kinetic energy = 1/2(mass of body)(speed of body)²
Kinetic energy = 1/2(mass of body)(speed of body)²2. Gravitational potential energy and work done
Ep = mgh
Potential energy = mass(gravitational field strength)(height)
6.3 Power
P= W/t = E/t
Power = work done over time = energy converted over time
SI Unit: watt (W)
One watt is defined as the rate of working or energy conversion of one joule per second
Tuesday, October 20, 2009
Physics: Chapter 5 - Turning Effect of Forces
5.1 Moments
The moment of a force is the product of the force and the perpendicular distance from the pivot to the line of action of the force.
Moment of a force = Fxd
Force(perpendicular distance from pivot) = Moments
Moment = turning effect of force
5.2 Principle of Moments
When a body is in equilibrium, the sum of clockwise moments about a pivot is equal to the sum of anticlockwise moments about the same pivot.
An object is said to satisfy the Principle of Moments when the force acting in clockwise motion is the same as force acting in anticlockwise motion on the same pivot.
Conditions for equilibrium:
5.4 Stability
Stability refers to the ability of an object to return to its original position after it has been tilted slightly.
Three cases of equilibrium:
The moment of a force is the product of the force and the perpendicular distance from the pivot to the line of action of the force.
Moment of a force = Fxd
Force(perpendicular distance from pivot) = Moments
Moment = turning effect of force
5.2 Principle of Moments
When a body is in equilibrium, the sum of clockwise moments about a pivot is equal to the sum of anticlockwise moments about the same pivot.
An object is said to satisfy the Principle of Moments when the force acting in clockwise motion is the same as force acting in anticlockwise motion on the same pivot.
Conditions for equilibrium:
- All forces acting on it are balanced (Resultant force is 0)
- The resultant moment about the pivot is 0 (Principle of Moments must apply)
The centre of gravity of an object is defined as the point through which its whole weight appears to act for any orientation of the object.The centre of gravity of a body is a single point through which its entire weight acts.
5.4 Stability
Stability refers to the ability of an object to return to its original position after it has been tilted slightly.
Three cases of equilibrium:
- Stable equilibrium
- Unstable equilibrium
- Neutral equilibrium
- The centre of gravity should be as low as possible
- The area of a base should be as wide as possible
Physics: Chapter 4 - Mass, Weight and Density
4.1 Mass and Weight
Mass is a measure of the amount of matter or substance in a body.
SI Unit: kilogram (kg)
Weight is a force and has direction.
SI Unit: newton (N)
Also know as gravity.
W = mg
Weight = mass(gravitational field strength)
Earth = 10kg-1
4.2 Inertia
Inertia of an object refers to the reluctance of the object to change its state of rest or motion.
4.3 Density
The density of a substance is defined as its mass per unit volume.
p = m/v
Density = mass/ volume
SI Unit: kilogram per cubic metre (kg m-3)
Mass is a measure of the amount of matter or substance in a body.
SI Unit: kilogram (kg)
Weight is a force and has direction.
SI Unit: newton (N)
Also know as gravity.
W = mg
Weight = mass(gravitational field strength)
Earth = 10kg-1
4.2 Inertia
Inertia of an object refers to the reluctance of the object to change its state of rest or motion.
4.3 Density
The density of a substance is defined as its mass per unit volume.
p = m/v
Density = mass/ volume
SI Unit: kilogram per cubic metre (kg m-3)
Physics: Chapter 3 - Forces and Pressure
3.1 Forces
3.2 Scalars and Vectors
Scalar quantities are physical quantities that have magnitude only
Vector quantities, however, are physical quantities that possess both magnitude and direction.
3.3 Forces and Motion
A force can cause:
Forces and Zero acceleration
Balanced forces and Newton's First Law
Unbalanced forces and Newton's Second Law
F = ma
Force = mass(acceleration)
SI Unit: Newton (N)
Weight is a force
W = mg
Newton's Third Law
3.4 Friction and its Effects
Friction always opposes motion between two surface in contact.
3.5 Pressure
Pressure = force / area
P = F/ A
SI Unit: newton per square metre (N m-2)
A force is a push or a pull that one object exerts on another.SI Unit: newton (N)
It produces or tends to produce motion, and stops or tends to stop motion.
3.2 Scalars and Vectors
Scalar quantities are physical quantities that have magnitude only
Vector quantities, however, are physical quantities that possess both magnitude and direction.
3.3 Forces and Motion
A force can cause:
- A stationary object to start moving
- A moving object to increase speed
- A moving object to decrease speed
- A moving object to change its direction of motion
Forces and Zero acceleration
For an object with zero acceleration, the different forces acting on it are balanced or add up to zero.
Balanced forces and Newton's First Law
Every object will continue in its state of rest or uniform motion in a straight line unless a resultant force acts on it to change its state.
Unbalanced forces and Newton's Second Law
When a resultant force acts on an object of constant mass, the object will accelerate and move in the direction of the resultant force.
The product of the mass and acceleration of the object is equal to the resultant force.
F = ma
Force = mass(acceleration)
SI Unit: Newton (N)
Weight is a force
W = mg
Newton's Third Law
For every action, there is an equal and opposite reaction, and these forces act on mutually opposite bodies.
3.4 Friction and its Effects
Friction always opposes motion between two surface in contact.
3.5 Pressure
Pressure = force / area
P = F/ A
SI Unit: newton per square metre (N m-2)
Physics: Chapter 2 - Kinematics
2.1 Distance, Time and Speed
Speed = Distance moved / time taken
v = d/t
Average speed = total distance travelled / total time taken
2.2 Speed, Velocity and Acceleration
Acceleration = change in velocity / time taken
2.3 Speed- Time graphs
Area under the speed time graph gives the distance moved.
2.4 Acceleration of free fall
Acceleration due to free fall (acceleration due to gravity) does not depend on the material, size or shape.
Speed = Distance moved / time taken
v = d/t
Average speed = total distance travelled / total time taken
2.2 Speed, Velocity and Acceleration
Acceleration = change in velocity / time taken
2.3 Speed- Time graphs
Area under the speed time graph gives the distance moved.
2.4 Acceleration of free fall
Acceleration due to free fall (acceleration due to gravity) does not depend on the material, size or shape.
Physics: Chapter 1 - Measurement
1.3 Measurement of Length
SI Unit: metre (m)
Instrument: Metre rule, tape measure, calipers, vernier calipers and micrometer screw gauge
1.3 Measurement of Time
SI Unit: seconds (s)
Instrument: Watch, stopwatch
SI Unit: metre (m)
Instrument: Metre rule, tape measure, calipers, vernier calipers and micrometer screw gauge
1.3 Measurement of Time
SI Unit: seconds (s)
Instrument: Watch, stopwatch
Physics: Chapter 17 - Practical Electricity
17.1 Uses of Electricity
Electricity is used for:
Advantages: Gives a cosy and relaxed atmosphere.
Disadvantages: Only a small % are converted to light energy while the rest are converted to heat energy.
Fluorescent lamps
Advantages: It is energy efficient.
Disadvantages: Costly. Mercury vapour in the lamp is toxic.
17.2 Measuring Electrical Energy
Electric Power P
P= VI
P= I2R
P= V2/R
SI Unit: watt (W)
Electric energy E
E= V2/R (t)
SI Unit: joule (J)
17.3 Dangers of Electricity
Faulty appliances or circuits can cause shock or fire.
Insulator can crack and break which exposed the conducting wires inside.
Exposed live wires can cause electric shock.
2) Overheating of cables
The higher resistance of thinner wires will produce more thermal heat that will damage the insulation and may cause a fire.
3) Damp conditions
Water provide a conducting path for a large current to flow. If can therefore shock a person to death.
17.4 Safe Use of Electricity at Home
a) Miniature circuit breaker MCB
b) Earth leakage circuit breaker ELCB
a) MCB prevents excessive current flow through in the circuit by tripping or breaking it.
It can be reset by switching it on again.
b) ELCB monitors the amount of current flowing from the live wire. Current in neutral wire should be the same as live wire. ELCB detects these small current leakages from the live wire to the earth wire.
2) Fuse
A fuse is a safety device included in an electrical circuit to prevent excessive current flow.
A fuse consists of a short thin piece of wire which becomes hot and melts when the current flowing through it is greater than its rated value.
Fuses should have a current rating just slightly higher than the current an electrical appliance will use under normal conditions.
3) Switches
Switches must be fitted onto the live wire so that switching off disconnects the high voltage from an appliance.
4) Plugs and sockets
Live wire : Brown
Earth wire: Green & Yellow
Neutral wire : Blue
5) Earthing
The earth wire is a low-resistance wire.
It is usually connected to the metal casing of the appliance.
6) Double insulation
Appliance that use a two-pin plug requires double insulation.
Electricity is used for:
- Heating (Kettle, iron, hotplate)
- Lighting (Filament lamps, fluorescent lamps)
- Electric Motors (Hair dryer, food mixer)
Advantages: Gives a cosy and relaxed atmosphere.
Disadvantages: Only a small % are converted to light energy while the rest are converted to heat energy.
Fluorescent lamps
Advantages: It is energy efficient.
Disadvantages: Costly. Mercury vapour in the lamp is toxic.
17.2 Measuring Electrical Energy
Electric Power P
P= VI
P= I2R
P= V2/R
SI Unit: watt (W)
Electric energy E
E= V2/R (t)
SI Unit: joule (J)
17.3 Dangers of Electricity
Faulty appliances or circuits can cause shock or fire.
- Damaged insulation (shock)
- Overheating of cables (fire)
- Damp Conditions (shock)
Insulator can crack and break which exposed the conducting wires inside.
Exposed live wires can cause electric shock.
2) Overheating of cables
The higher resistance of thinner wires will produce more thermal heat that will damage the insulation and may cause a fire.
3) Damp conditions
Water provide a conducting path for a large current to flow. If can therefore shock a person to death.
17.4 Safe Use of Electricity at Home
- Circuit breakers
- Fuse
- Correct placement of switch in the circuit
- The three-pin plug
- Earth wire
- Double insulation of certain appliances
a) Miniature circuit breaker MCB
b) Earth leakage circuit breaker ELCB
a) MCB prevents excessive current flow through in the circuit by tripping or breaking it.
It can be reset by switching it on again.
b) ELCB monitors the amount of current flowing from the live wire. Current in neutral wire should be the same as live wire. ELCB detects these small current leakages from the live wire to the earth wire.
2) Fuse
A fuse is a safety device included in an electrical circuit to prevent excessive current flow.
A fuse consists of a short thin piece of wire which becomes hot and melts when the current flowing through it is greater than its rated value.
Fuses should have a current rating just slightly higher than the current an electrical appliance will use under normal conditions.
3) Switches
Switches must be fitted onto the live wire so that switching off disconnects the high voltage from an appliance.
4) Plugs and sockets
Live wire : Brown
Earth wire: Green & Yellow
Neutral wire : Blue
5) Earthing
The earth wire is a low-resistance wire.
It is usually connected to the metal casing of the appliance.
6) Double insulation
Appliance that use a two-pin plug requires double insulation.
Monday, October 19, 2009
Physics: Chapter 15 - Current Electricity
15.1 Electric Current
Electric current is formed by moving electrons.
Conventional current and electron flow
Electron flow: Electrons moving from a negatively charged end to a positively charged end
Conventional current flow: Positive charges flowing from a positively charged end to a negatively charged end.
Measurement of electric current
Current = Charge / time
SI unit: ampere
Symbol: A
Instrument to measure: Ammeter
Ammeter must be connected in series in an electric circuit. Flow from + to - terminal.

Electric Circuit symbols

15.2 Electromotive Force and Potential Difference
E = W/Q
E.M.F. of the power supply = work done/ charge
SI unit: joules per coulomb (J C-1) or Volt (V)
Instrument to measure: Voltmeter

Potential Difference
V = W/Q
Potential difference = Work done/Charge
SI unit: volt (V)
15.3 Resistance
Resistance is a measure of how difficult it is for an electric current to pass through a material.
R = V/I
Resistance = Potential difference/current
SI unit: ohm (Ω)
Instruments: Fixed resistors or rheostats


Ohm's Law
15.4 Resistivity
Resistance of a conductor depends on
resistivity = resistance(cross-sectional area)/length
Electric current is formed by moving electrons.
Conventional current and electron flow
Electron flow: Electrons moving from a negatively charged end to a positively charged end
Conventional current flow: Positive charges flowing from a positively charged end to a negatively charged end.
Measurement of electric current
An electric current I is a measure of the rate of flow of electric charge Q through a given cross section of a conductor.I = Q/t
Current = Charge / time
SI unit: ampere
Symbol: A
Instrument to measure: Ammeter
Ammeter must be connected in series in an electric circuit. Flow from + to - terminal.

Electric Circuit symbols

15.2 Electromotive Force and Potential Difference
The electric force (e.m.f.) of an electrical energy source is defined as the work done by the source in driving a unit charge round a complete circuit.
E = W/Q
E.M.F. of the power supply = work done/ charge
SI unit: joules per coulomb (J C-1) or Volt (V)
Instrument to measure: Voltmeter

Potential Difference
The potential difference (p.d.) between two points in an electric circuit is defined as the amount of electrical energy converted to other forms of energy when one coulomb of positive charge passes between the two points.
V = W/Q
Potential difference = Work done/Charge
SI unit: volt (V)
E.M.F. is provided by a source of electric energy.
P.D. refers to the electrical energy converted to other forms by a circuit component.
15.3 Resistance
Resistance is a measure of how difficult it is for an electric current to pass through a material.
The resistance R of a component is defined as the ratio of the potential difference V across it to the current I flowing through it.
R = V/I
Resistance = Potential difference/current
SI unit: ohm (Ω)
Instruments: Fixed resistors or rheostats


Ohm's Law
The current passing through a metallic conductor is directly proportional to the potential difference across its ends, provided the physical conditions (such as temperature) are constant.
15.4 Resistivity
Resistance of a conductor depends on
- its length
- its cross-sectional are or thickness of the wire
- the type of material
resistivity = resistance(cross-sectional area)/length
Physics: Chapter 14 - Static Electricity
14.1 Electrostatics
It is the study of static electric charges.
Electrostatic charging by friction
Some objects acquire electric charges by rubbing them together to transfer electrons from one object to another.
There are two types of charges: positive and negative.
Like charges repel, unlike charges attract.
Measurement of electric charge
Electric charge is measured in coulombs, C.
14.2 Electric Field
An electric field is a region where an electric charge experiences an electric force.
The direction of the field is defines as the direction of the force on a small positive charge.
The strength of an electric field is indicated by how close the field lines are to each other.

It is the study of static electric charges.
Electrostatic charging by friction
Some objects acquire electric charges by rubbing them together to transfer electrons from one object to another.
There are two types of charges: positive and negative.
Like charges repel, unlike charges attract.
Measurement of electric charge
Electric charge is measured in coulombs, C.
14.2 Electric Field
An electric field is a region where an electric charge experiences an electric force.
The direction of the field is defines as the direction of the force on a small positive charge.
The strength of an electric field is indicated by how close the field lines are to each other.

Subscribe to:
Comments (Atom)
